Multiple Choice
Which one of the following is the correct electron configuration for a ground-state nitrogen atom?
A) 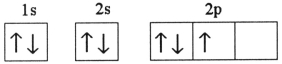
B) 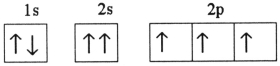
C) 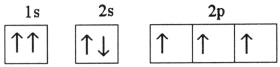
D) 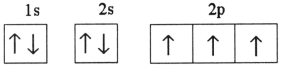
E) None of the above is correct.
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q10: Blackbody radiation is the emission of light
Q47: Each d-subshell can accommodate a maximum of
Q59: The ground state electron configuration of scandium
Q65: The frequency of a photon that has
Q88: _-orbitals are spherically symmetrical.<br>A)s<br>B)p<br>C)d<br>D)f<br>E)g
Q99: The energy of a photon that has
Q137: All of the subshells in a given
Q158: If a hydrogen atom electron jumps from
Q171: What is the frequency of light (s<sup>-1</sup>)that
Q178: The element that corresponds to the electron