Multiple Choice
Which electron configuration denotes an atom in its ground state?
A) 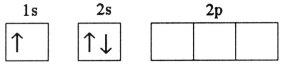
B) 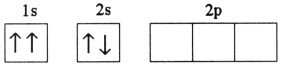
C) 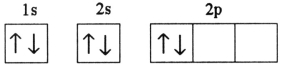
D) 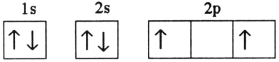
E) 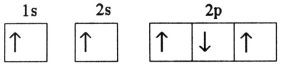
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q7: Elements in group _ have a np<sup>5</sup>
Q12: Which one of the following configurations depicts
Q13: Which one of the following represents an
Q15: Which electron configuration represents a violation of
Q44: The 4d subshell in the ground state
Q60: The square of Schrodinger's wave equation is
Q72: There are _ orbitals in the third
Q92: Of the following transitions in the Bohr
Q103: Which one of the following is not
Q135: There are _ unpaired electrons in a