Multiple Choice
Which electron configuration represents a violation of Hund's rule for an atom in its ground state?
A) 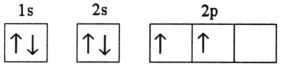
B) 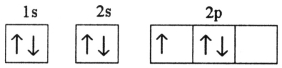
C) 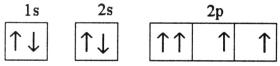
D) 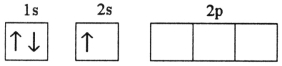
E) 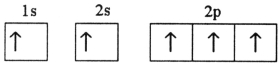
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q1: What color of visible light has the
Q22: What is the frequency of light (s<sup>-1</sup>)that
Q25: The de Broglie wavelength of a 6.0
Q71: Of the following, _ radiation has the
Q121: In a hydrogen atom, an electron in
Q123: The electron density of the 2s orbital
Q125: [Ne]3s<sup>2</sup>3p<sup>3</sup> is the electron configuration of a(n)_
Q133: [Ar]4s<sup>2</sup>3d<sup>10</sup>4p<sup>3</sup> is the electron configuration of a(n)_
Q145: When the electron in a hydrogen atom
Q183: The _ quantum number defines the shape