Multiple Choice
The energy of a photon that has a wavelength of 13.2 nm is __________ J.
A) 9.55 × 10-25
B) 1.62 × 10-17
C) 1.99 × 10-25
D) 4.42 × 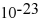
E) 1.51 × 10-17
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q35: Which of the following is a valid
Q37: What is the wavelength of light (nm)that
Q42: In a p<sub>x</sub> orbital, the subscript x
Q44: Of the following transitions in the Bohr
Q85: What color of visible light has the
Q156: The ground state electron configuration of copper
Q159: The angular momentum quantum number is 3
Q163: The correct ground-state electron configuration for silver
Q179: The ground-state electron configuration of _ is
Q180: The n = 8 to n =