Multiple Choice
Using Bohr's equation for the energy levels of the electron in the hydrogen atom, determine the 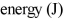 of an electron in the n = 4 level.
of an electron in the n = 4 level.
A) -1.36 × 10-19
B) -5.45 × 10-19
C) -7.34 × 1018
D) -1.84 × 10-29
E) +1.84 × 10-29
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q52: What is the wavelength of light (nm)that
Q54: The complete electron configuration of gallium, element
Q55: When the electron in a hydrogen atom
Q57: Which electron configuration represents a violation of
Q59: What is the wavelength (angstroms)of a photon
Q60: Which one of the following represents an
Q70: The 3p subshell in the ground state
Q77: Elements in group _ have a np<sup>6</sup>
Q159: An NMR spectrum results from photon irradiation
Q185: The ground state electron configuration of Ga