Multiple Choice
The hybridization of the oxygen atom labeled y in the structure below is __________. The C-O-H bond angle is __________. 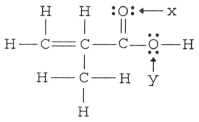
A) sp, 180°
B) sp2, 109.5°
C) sp3, 109.5°
D) sp3d2, 90°
E) sp, 90°
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q19: The total number of π bonds in
Q68: The basis of the VSEPR model of
Q68: The hybrid orbital set used by the
Q71: The molecular geometry of the SF<sub>6</sub> molecule
Q77: Of the molecules below, only _ is
Q78: The hybrid orbital set used by the
Q80: Of the following, only _ appears to
Q91: The hybrid orbitals used for bonding by
Q99: The hybridization of the central atom in
Q166: The Cl-Si-Cl bond angle in the SiCl<sub>2</sub>F<sub>2</sub>