Multiple Choice
The Lewis structure of carbon dioxide is given below. The hybridization of the carbon atom in carbon dioxide is __________. 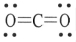
A) sp3
B) sp2
C) sp
D) sp2d
E) sp2d2
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q26: The hybridization of orbitals on the central
Q57: The hybridizations of nitrogen in NF<sub>3</sub> and
Q66: The bond order of a homonuclear diatomic
Q96: A covalent bond in which overlap regions
Q105: The angles between sp<sup>2</sup> orbitals are _.<br>A)45°<br>B)180°<br>C)90°<br>D)109.5°<br>E)120°
Q108: Nitrogen is colorless because the minimum energy
Q127: ClF<sub>3</sub> has "T-shaped" geometry. There are _
Q131: The bond angles marked a, b, and
Q134: The O-C-O bond angle in the CO<sub>3</sub><sup>2-</sup>
Q142: PCl<sub>5</sub> has _ electron domains and a