Multiple Choice
There is/are __________ π bond(s) in the molecule below. 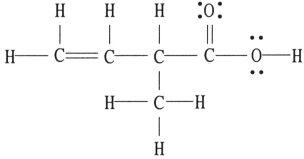
A) 0
B) 1
C) 2
D) 4
E) 16
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q21: Molecular Orbital theory correctly predicts diamagnetism of
Q25: Of the following species,_ will have bond
Q39: Three molecules have similar electron domains,but different
Q47: The molecular geometry of the CS<sub>2</sub> molecule
Q94: An electron domain consists of _. <br>a.a
Q124: The molecular geometry of the PF<sub>4</sub><sup>+</sup> ion
Q153: The hybridization of the oxygen atom labeled
Q160: The hybrid orbitals used for bonding by
Q161: According to VSEPR theory, if there are
Q179: Of the following molecules,only _ is polar.<br>A)CCl<sub>4</sub><br>B)BCl<sub>3</sub><br>C)NCl<sub>3</sub><br>D)BeCl<sub>2</sub><br>E)Cl<sub>2</sub>