Multiple Choice
There is/are __________ π bond(s) in the molecule below. 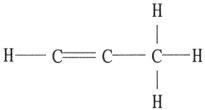
A) 7
B) 6
C) 2
D) 1
E) 0
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q8: The central Xe atom in the XeF<sub>4</sub>
Q11: A typical double bond consists of _.<br>A)three
Q70: In a typical multiple bond, the σ
Q90: Using the VSEPR model, the molecular geometry
Q92: The hybridization of the terminal carbons in
Q94: According to MO theory, overlap of two
Q96: Using the VSEPR model, the electron-domain geometry
Q123: Which of the molecules has a see-saw
Q151: The 1s hydrogen orbital overlaps with the
Q159: Three monosulfur fluorides are observed: SF<sub>2</sub>,SF<sub>4</sub>,and SF<sub>6</sub>.Of