Multiple Choice
A gas vessel is attached to an open-end manometer filled with a nonvolatile liquid of density 0.993 g/mL as shown below. 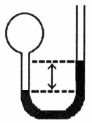 The difference in heights of the liquid in the two sides of the manometer is 32.3 mm when the atmospheric pressure is 765 mm Hg. Given that the density of mercury is 13.6 g/mL, the pressure of the enclosed gas is __________ atm.
The difference in heights of the liquid in the two sides of the manometer is 32.3 mm when the atmospheric pressure is 765 mm Hg. Given that the density of mercury is 13.6 g/mL, the pressure of the enclosed gas is __________ atm.
A) 1.05
B) 1.01
C) 0.976
D) 0.993
E) 1.08
Correct Answer:

Verified
Correct Answer:
Verified
Q21: If the temperature is lowered from 60
Q39: A sample of gas (1.3 mol)occupies _
Q45: A gas originally at 27°C and 1.00
Q74: The van der Waals equation for real
Q116: The deviation from ideal behavior of a
Q127: The effusion rate of a gas is
Q142: The average kinetic energy of the particles
Q142: The volume of a sample of gas
Q143: A gas at a pressure of 10.0
Q174: A vessel contained N<sub>2</sub>,Ar,He,and Ne.The total pressure