Multiple Choice
Which one of the following substances will not have hydrogen bonding as one of its intermolecular forces?
A) 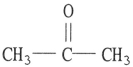
B) 
C) 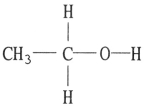
D) 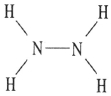
E) 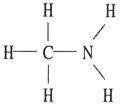
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q8: Which type of liquid crystal is colored
Q11: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB2701/.jpg" alt=" -The heating curve
Q26: Which one of the following exhibits dipole-dipole
Q30: Ethanol (C<sub>2</sub>H<sub>5</sub>OH)melts at -114°C. The enthalpy of
Q45: The intermolecular force(s)responsible for the fact that
Q62: The heating curve shown was generated by
Q63: In the _ liquid crystalline phase, the
Q76: Viscosity is _.<br>A)the "skin" on a liquid
Q89: Which statements about viscosity are true?<br>(i)Viscosity increases
Q115: On the phase diagram shown above,segment _