Multiple Choice
Based on molecular mass and dipole moment of the five compounds in the table below, which should have the highest boiling point? 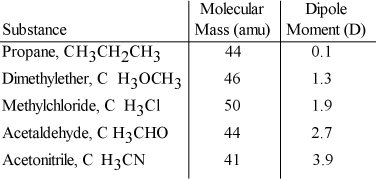
A) CH3CH2CH3
B) CH3OCH3
C) CH3Cl
D) CH3CHO
E) CH3CN
Correct Answer:

Verified
Correct Answer:
Verified
Q12: Elemental iodine (I<sub>2</sub>)is a solid at room
Q19: _ is the energy required to expand
Q28: For a given substance that exhibits liquid-crystalline
Q64: London Dispersion Forces tend to _ in
Q80: When the phase diagram for a substance
Q113: Hydrogen bonding is a special case of
Q113: Which of the following has dispersion forces
Q115: Which compound has the strongest intermolecular forces?<br>A)CBr<sub>4</sub><br>B)C<sub>12</sub>H<sub>26</sub><br>C)CI<sub>4</sub><br>D)N<sub>2</sub><br>E)O<sub>2</sub>
Q116: The phase diagram of a substance is
Q122: The boiling points of normal hydrocarbons are