Multiple Choice
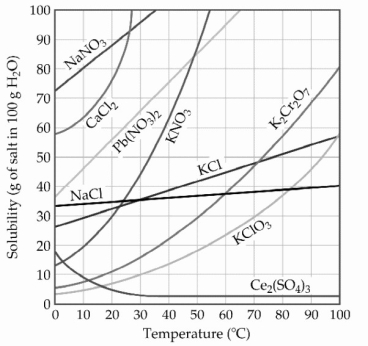
-A sample of potassium chlorate (15.0 g) is dissolved in 201 g of water at 70 °C, with precautions taken to avoid evaporation of any water. The solution is cooled to 30.0 °C and no precipitate is observed. This solution is ________.
A) hydrated
B) miscible
C) saturated
D) unsaturated
E) supersaturated
Correct Answer:

Verified
Correct Answer:
Verified
Q145: A supersaturated solution _.<br>A)is one with more
Q146: A solution contains 150.8 grams of NaCl
Q147: A solution is prepared by dissolving 15.0
Q148: Calculate the molality of a 10.0% (by
Q149: A solution is prepared by dissolving calcium
Q151: Calculate the molality of a 27.0% (by
Q151: The concentration of urea (MW = 60.0
Q152: Which of the following substances is more
Q153: A solution contains 30 ppm of benzene.
Q154: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB2701/.jpg" alt=" -A sample of