Multiple Choice
A- is a weak base. Which equilibrium corresponds to the equilibrium constant Ka for HA?
A) HA (aq) + H2O (l) 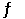 H2A+ (aq) + OH- (aq)
H2A+ (aq) + OH- (aq)
B) A- (aq) + H3O+ (aq) 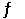 HA (aq) + H2O (l)
HA (aq) + H2O (l)
C) HA (aq) + H2O (l) 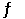 H3O+ (aq) + A- (aq)
H3O+ (aq) + A- (aq)
D) A- (aq) + H2O (l) 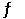 HA (aq) + OH- (aq)
HA (aq) + OH- (aq)
E) A- (aq) + OH- (aq) 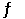 HOA2- (aq)
HOA2- (aq)
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q19: A 0.0035 M aqueous solution of a
Q59: The acid-dissociation constant at 25.0°C for hypochlorous
Q61: A 0.1 M aqueous solution of _
Q62: The pH of an aqueous solution at
Q63: The K<sub>a</sub> of hydrofluoric acid (HF)at 25.0
Q65: Determine the pH of a 0.35 M
Q68: Using the data in the table, which
Q69: Of the acids in the table below,
Q89: A solution of acetic acid is 2.0%
Q138: Of the following acids,_ is not a