Multiple Choice
Using the data in the table, which of the conjugate bases below is the strongest base? 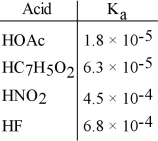
A) OAc-
B) C7H5O2-
C) NO2-
D) F-
E) OAc- and C7H5O2-
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q46: The molar concentration of hydronium ion in
Q63: Ammonia is a _.<br>A)weak acid<br>B)strong base<br>C)weak base<br>D)strong
Q79: Which of the following aqueous solutions has
Q80: Of the compounds below, a 0.1 M
Q82: K<sub>b</sub> for NH<sub>3</sub><sub> </sub>is 1.8 × 10<sup>-5</sup>.
Q83: What is the concentration (in M)of hydronium
Q87: Calculate the concentration (in M)of hydroxide ions
Q88: Calculate the pH of a solution at
Q94: Of the following substances,an aqueous solution of
Q112: The base-dissociation constant, K<sub>b</sub>, for pyridine, C<sub>5</sub>H<sub>5</sub>N,