Multiple Choice
Using the data in the table, which of the conjugate bases below is the weakest base? 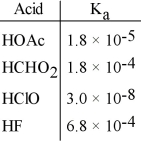
A) OAc-
B) CHO2-
C) ClO-
D) F-
E) OAc- and CHO2-
Correct Answer:

Verified
Correct Answer:
Verified
Q3: The acid-dissociation constant, K<sub>a</sub>, for gallic acid
Q35: The K<sub>a</sub> of acetic acid (HC<sub>2</sub>H<sub>3</sub>O<sub>2</sub>)is 1.8
Q36: What is the pH of a 0.035
Q40: HA is a weak acid. Which equilibrium
Q41: Calculate the molarity of hydroxide ion in
Q42: Calculate the pH of a 0.0787 M
Q43: Nitric acid is a strong acid. This
Q68: A solution of ammonia is 2.0% ionized
Q105: The conjugate base of HSO<sub>4</sub><sup>-</sup> is _.<br>A)OH<sup>-</sup><br>B)H<sub>2</sub>SO<sub>4</sub><br>C)SO<sub>4</sub><sup>2-</sup><br>D)HSO<sub>4</sub><sup>+</sup><br>E)H<sub>3</sub>SO<sub>4</sub><sup>+</sup>
Q123: In the reaction<br>BF<sub>3</sub> + F<sup>-</sup> → BF<sub>4</sub><sup>-</sup><br>BF<sub>3</sub>