Multiple Choice
Using the data in the table, which of the conjugate acids below is the weakest acid? 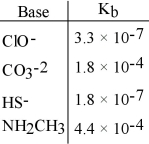
A) HClO
B) HCO3-
C) H2S
D) NH3CH3+
E) H2S and HClO
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q3: In the gas phase reaction below, NH<sub>3</sub>
Q4: Calculate the pH of 0.586 M anilinium
Q10: K<sub>b</sub> for NH<sub>3</sub> is 1.8 × 10<sup>-5</sup>.
Q11: In which of the following aqueous solutions
Q13: Classify the following compounds as weak acids
Q24: Which of the following ions will act
Q28: A solution of formic acid is 3.0%
Q38: An acid containing the COOH group is
Q56: Classify the following compounds as weak bases
Q119: K<sub>a</sub> for HCN is 4.9 × 10<sup>-10</sup>.