Multiple Choice
Using the data in the table, which of the conjugate acids below is the strongest acid? 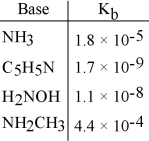
A) NH4+
B) C5H5NH+
C) H3NOH+
D) NH3CH3+
E) NH4+ and NH3CH3+
Correct Answer:

Verified
Correct Answer:
Verified
Q9: The molar concentration of hydroxide ion in
Q84: K<sub>a</sub> for HF is 7.0 × 10<sup>-4</sup>.K<sub>b</sub>
Q100: Classify the following compounds as weak acids
Q118: What is the conjugate acid of NH<sub>3</sub>?<br>A)NH<sub>3</sub><br>B)NH<sub>2</sub><sup>+</sup><br>C)NH<sub>3</sub><sup>+</sup><br>D)NH<sub>4</sub><sup>+</sup><br>E)NH<sub>4</sub>OH
Q126: Which one of the following is a
Q127: Which one of the following is the
Q128: Determine the pOH of a 0.35 M
Q130: Which solution below has the highest concentration
Q133: Using the data in the table, which
Q134: An aqueous solution at 25.0°C contains [H<sup>+</sup>]