Multiple Choice
Using the data in the table, which of the conjugate acids below is the weakest acid? 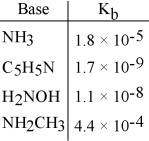
A) NH4+
B) C5H5NH+
C) H3NOH+
D) NH3CH3+
E) NH4+ and NH3CH3+
Correct Answer:

Verified
Correct Answer:
Verified
Q1: The magnitude of K<sub>w</sub> indicates that _.<br>A)water
Q26: The K<sub>a</sub> of hypochlorous acid (HClO)is 3.00
Q57: A Br∅nsted-Lowry base is defined as a
Q57: The hydride ion, H<sup>-</sup>, is a stronger
Q105: A Br∅nsted-Lowry acid is defined as a
Q110: An aqueous solution contains 0.050 M of
Q111: The pH of a 0.60 M aqueous
Q113: What is the pH of an aqueous
Q119: A 0.15 M aqueous solution of the
Q129: The conjugate acid of HSO<sub>4</sub><sup>-</sup> is _.<br>A)SO<sub>4</sub><sup>2-</sup><br>B)H<sub>2</sub>SO<sub>4</sub><br>C)HSO<sub>4</sub><sup>+</sup><br>D)H<sup>+</sup><br>E)HSO<sub>3</sub><sup>+</sup>