Multiple Choice
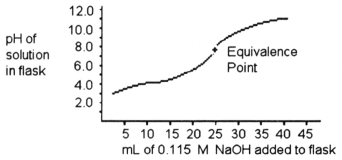
-A 25.0 mL sample of a solution of a monoprotic acid is titrated with a 0.115 M NaOH solution. The titration curve above was obtained. The concentration of the monoprotic acid is about ________ mol/L.
A) 25.0
B) 0.0600
C) 0.240
D) 0.120
E) 0.100
Correct Answer:

Verified
Correct Answer:
Verified
Q24: Which one of the following is not
Q25: Human blood is _.<br>A)neutral<br>B)very basic<br>C)slightly acidic<br>D)very acidic<br>E)slightly
Q26: The solubility of lead (II)chloride (PbCl<sub>2</sub>)is 1.6
Q27: Calculate the pH of a solution prepared
Q28: Calculate the percent ionization of formic acid
Q29: Suppose you have just added 100.0 ml
Q31: What is the pH of a buffer
Q33: Which of the following could be added
Q35: A solution of NaF is added dropwise
Q36: The pH of a solution prepared by