Multiple Choice
Which one of the following correctly indicates the relationship between the entropy of a system and the number of different arrangements, W, in the system?
A) S = kW
B) S = 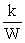
C) S = 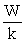
D) S = klnW
E) S = Wk
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q44: The equilibrium constant for a reaction is
Q47: The value of ΔG° at 100.0°C for
Q47: The combustion of ethene in the presence
Q50: Given the following table of thermodynamic data,
Q51: The equilibrium position corresponds to which letter
Q52: The normal boiling point of water is
Q53: Consider the reaction: Ag<sup>+</sup> (aq)+ Cl<sup>-</sup> (aq)→
Q82: The value of ΔS° for the oxidation
Q104: For the reaction 2 C<sub>4</sub>H<sub>10</sub> (g)+ 13
Q113: The value of ΔS° for the formation