Multiple Choice
Given the thermodynamic data in the table below, calculate the equilibrium constant (at 298 K) for the reaction: 2SO2 (g) + O2 (g) 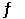 2SO3 (g)
2SO3 (g) 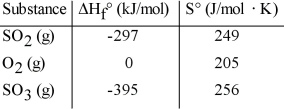
A) 2.37 × 1024
B) 1.06
C) 1.95
D) 3.82 × 1023
E) More data are needed.
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q21: The value of ΔS° for the oxidation
Q33: The vaporization of a substance at its
Q58: The value of ΔG° at 25 °C
Q61: The value of ΔS° for the decomposition
Q91: The value of ΔS° for the formation
Q112: For a given reaction, ΔH = +35.5
Q114: The first law of thermodynamics can be
Q115: The equilibrium constant for the following reaction
Q119: Which one of the following processes produces
Q125: The value of ΔS° for the decomposition