Multiple Choice
The mass of a proton is 1.00728 amu and that of a neutron is 1.00867 amu. What is the mass defect (in amu) of a 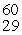 Ni nucleus? (The mass of a nickel-60 nucleus is 59.9308 amu.)
Ni nucleus? (The mass of a nickel-60 nucleus is 59.9308 amu.)
A) 0.5449
B) 1.2374
C) 0.5491
D) 28.7930
E) 1.3066
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q10: High speed electrons emitted by an unstable
Q11: What isotope of what element is produced
Q68: The missing product in this reaction would
Q69: The missing product from this reaction is
Q70: The mass of a proton is 1.00728
Q71: The mass of a proton is 1.00728
Q75: In the nuclear transmutation represented by <img
Q76: Which of the following correctly represents the
Q163: Alpha decay produces a new nucleus whose
Q178: The initial element used to make cobalt-60