Multiple Choice
This reaction is an example of __________. 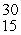 P + _____ →
P + _____ → 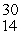 Si
Si
A) alpha decay
B) beta decay
C) positron decay
D) electron capture
E) gamma emission
Correct Answer:

Verified
Correct Answer:
Verified
Q3: The amount of fissionable material necessary to
Q20: The major type of cancer caused by
Q29: The belt of nuclear stability ends with
Q30: On average,_ neutrons are produced by every
Q44: The half-life for the beta decay of
Q73: Atoms containing radioactive nuclei are called _.<br>A)radionuclides<br>B)radioisotopes<br>C)nucleons<br>D)nuclides<br>E)radioisophores
Q80: The half-life of <sup>218</sup>Po is 3.1 minutes.How
Q103: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB1822/.jpg" alt=" e represents _."
Q112: Which one of the following is a
Q146: In the formula k = 0.693/t<sub>1/2</sub>,k is