Multiple Choice
The mass of a proton is 1.00728 amu and that of a neutron is 1.00867 amu. What is the mass defect (in amu) of a 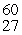 Co nucleus? (The mass of a cobalt-60 nucleus is 59.9338 amu.)
Co nucleus? (The mass of a cobalt-60 nucleus is 59.9338 amu.)
A) 27.7830
B) 0.5489
C) 0.5405
D) 0.0662
E) 0.4827
Correct Answer:

Verified
Correct Answer:
Verified
Q33: The beta decay of cesium-137 has a
Q36: The half-life of <sup>223</sup>Ra is 11.4 days.How
Q82: The half-life of carbon-11 is 20.3 minutes.How
Q111: The SI unit of an absorbed dose
Q123: What is the missing product from this
Q126: The decay of a radionuclide with a
Q127: The reaction shown below is responsible for
Q132: In balancing the nuclear reaction <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB1822/.jpg"
Q135: The half-life for beta decay of strontium-90
Q150: The basis for the carbon-14 dating method