Multiple Choice
Carbon-11 decays by positron emission: 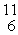 C →
C → 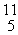 B +
B + 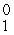 e The decay occurs with a release of 2.87 × 1011 J per mole of carbon-11. When 4.00 g of carbon-11 undergoes this radioactive decay, __________ g of mass is converted to energy.
e The decay occurs with a release of 2.87 × 1011 J per mole of carbon-11. When 4.00 g of carbon-11 undergoes this radioactive decay, __________ g of mass is converted to energy.
A) 1.16 × 10-3
B) 3.48 × 105
C) 1.16 × 10-6
D) 8.62 × 102
E) 1.28 × 10-2
Correct Answer:

Verified
Correct Answer:
Verified
Q2: The half-life of a radionuclide _.<br>A)is constant<br>B)gets
Q17: _ discovered radioactivity.
Q27: Which one of the following is true?<br>A)Some
Q85: What happens to the mass number and
Q90: This reaction is an example of _.
Q94: Potassium-40 decays to argon-40 with a half-life
Q99: The mass of a proton is 1.673
Q100: In the nuclear transmutation represented by <img
Q153: What drives the turbine in a nuclear
Q168: Bombardment of uranium-238 with a deuteron (hydrogen-2)generates