Multiple Choice
Which of the following best represents an sp2 hybridized atomic orbital of carbon which overlaps with the 1s atomic orbital of hydrogen to form a C-H bonding molecular orbital in ethene, H2C=CH2 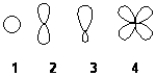
A) 1
B) 2
C) 3
D) 4
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q50: Which of the following species possesses a
Q81: How many electrons can the shell with
Q91: Which of the following molecules is
Q92: How many electrons are there in the
Q94: The following molecule contains an _functional group.
Q97: Which of the following bonds has the
Q98: Which of the following best represents the
Q99: How many electrons are there in the
Q100: The following molecule is classified as a
Q101: Draw bond-line structures of all of