Multiple Choice
Which of the following best represents an sp3 hybridized atomic orbital containing the lone pair of electrons of ammonia, NH3? 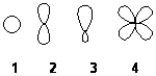
A) 1
B) 2
C) 3
D) 4
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q5: Convert the following structure into a bond-line
Q6: Which of the following molecules has
Q7: Which of the circled bonds is the
Q9: Which of the following resonance structures makes
Q11: Which of the following species has an
Q12: Which of the following species has an
Q13: Which of the following bonds is a
Q14: What is the approximate value of
Q15: Which of the following molecules has a
Q93: Draw bond-line structures of all of the