Essay
Use curved arrows to show the movement of pairs of electrons in the following reaction between a Lewis acid and a Lewis base, and show the structure of the product. 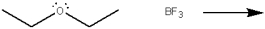
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q15: Which of the following terms describes the
Q69: Which set of curved arrows accounts for
Q70: The pK<sub>a</sub> of HCl is -7. What
Q70: Which of the following has the highest
Q71: Which atom in the following structure is
Q72: Which species is the conjugate acid in
Q73: Which of the following terms describes the
Q76: Consider the following reaction coordinate diagram. <img
Q78: The following is generic depiction of a
Q79: Provide the equation for the acid dissociate