Multiple Choice
Which of the following represents the transition state of the rate-determining step in the reaction between tert-butyl bromide and methanol leading to elimination? 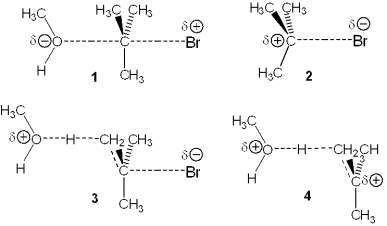
A) 1
B) 2
C) 3
D) 4
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q48: What is the major elimination product obtained
Q49: The rate law for the following reaction
Q50: What is the best choice of reagent
Q51: The first step in the mechanism for
Q52: What is the major organic product obtained
Q55: Classify each of the following species ,Place
Q56: What is the major organic product obtained
Q57: Which of the following represents the transition
Q58: In the following reaction the cyanide ion
Q62: The reaction of methyl iodide with sodium