Short Answer
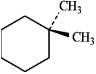
For each of the compounds below tell how many signals you would expect the molecule to have in its normal, broadband hydrogen-decoupled 13C NMR spectra. Enter the numerical value in the blank provided to the left of the structure.
-_______ 
Correct Answer:

Verified
Correct Answer:
Verified
Q9: Which of the following compounds gives a
Q11: What is the hydrogen deficiency index for
Q14: Which of the following combinations of peaks
Q51: What is the hydrogen deficiency index for
Q64: Which C<sub>9</sub>H<sub>10</sub>O compound gives the following <sup>1</sup>H
Q65: Which C<sub>4</sub>H<sub>9</sub>Br compound gives a triplet at
Q66: Consider the compound shown below. <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB1813/.jpg"
Q71: Which C<sub>6</sub>H<sub>12</sub>O<sub>2</sub> compound gives the following <sup>1</sup>H
Q74: Which of the protons in the following
Q76: How many signals appear in the proton-decoupled