Essay
Explain why the molecule shown below has a lower boiling point than CH3CH2CH2CH3. 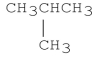
Correct Answer:

Verified
CH3CH2CH2CH3 has greater van der W...View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Correct Answer:
Verified
CH3CH2CH2CH3 has greater van der W...
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Related Questions
Q11: Draw the structure of N-ethyl-5-methyl-3-hexanamine.
Q20: Draw the most stable conformer of cis-1-isopropyl-2-methylcyclohexane.
Q34: Which compound is more soluble in water?
Q44: Draw an acceptable structure for 3-ethyl-3-methylhexane.
Q64: Provide the IUPAC name of the compound.
Q69: Provide the common name of the compound.
Q72: Provide an acceptable name for the compound
Q77: Provide the chair structure of cis-decalin,two cis-fused
Q79: Draw an acceptable structure for 4-t-butyloctane.
Q113: What is the strongest intermolecular force present