Essay
The compound below contains an asymmetric center at nitrogen. Why can't individual stereoisomers of this compound be isolated at room temperature? 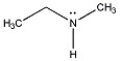
Correct Answer:

Verified
Nitrogen has a pair of nonbond...View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q30: How many asymmetric centers are present in
Q31: How many diastereomers exist for the compound
Q32: Which of the following terms best describes
Q34: Label each asymmetric center in the compound
Q36: Identify the following compounds as R or
Q37: Is the molecule shown chiral? Is it
Q39: Identify the compound(s)with asymmetric centers.<br>A) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB1830/.jpg"
Q40: Which of the following compounds is an
Q41: Draw the structure of (2R,3S)-dichloropentane.Take particular care
Q94: How many stereoisomers exist with the following