Multiple Choice
Which of the following pairs are resonance structures? 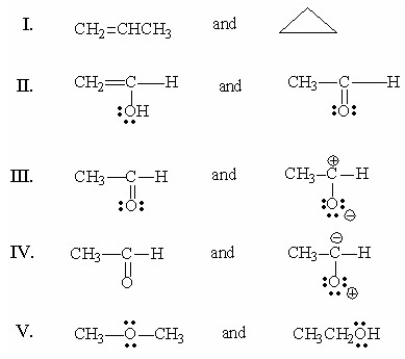
A) I
B) II
C) III
D) IV
E) V
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q19: What diene and dienophile should be used
Q20: What diene and dienophile should be used
Q24: Which of the following is an aromatic
Q25: Provide the structure of the major product
Q26: Provide the major organic product. <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB1830/.jpg"
Q27: What diene and dienophile should be used
Q28: What diene and what dieneophile would be
Q37: Draw (Z)-1,3-hexadiene in its s-trans conformation.
Q86: Which of the following compounds contains the
Q106: Show how the p orbitals overlap to