When 2,2-Dimethyloxirane (See Figure Below)undergoes Anionic Polymerization, the Nucleophile Attacks
Essay
When 2,2-dimethyloxirane (see figure below)undergoes anionic polymerization, the nucleophile attacks the least substituted carbon of the epoxide, but when it undergoes cationic polymerization, the nucleophile attacks the most substituted carbon of the epoxide. Explain why this is so. 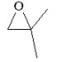
Correct Answer:

Verified
In anionic polymerization, the nucleophi...View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q9: List the two main groups of polymers
Q21: Describe what plasticizers are and give an
Q54: Identify the monomers that undergo cationic polymerization.<br>A)styrene<br>B)methyl
Q55: What is the structure of the monomer
Q56: What is the structure of the monomer
Q57: Propose a mechanism for the following polymerization
Q73: Which of the following is more likely
Q85: A substituted ethylene polymer in which the
Q99: From which of the following monomers is
Q106: Draw the structure of Nylon 66.