Multiple Choice
Use the periodic table below to answer the following questions. 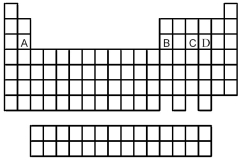
-Which is the correct formula of the binary fluoride of element B?
A) BF2
B) BF3
C) BF5
D) BF6
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q257: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB4940/.jpg" alt=" -Which of the
Q258: Ethane is an example of<br>A)a compound.<br>B)an element.<br>C)a
Q259: How many protons (p)and neutrons (n)are in
Q260: To the nearest whole number,the number of
Q261: A cake is an example of<br>A)a compound.<br>B)an
Q263: Which one of the following elements is
Q264: The subatomic particles contained in the nucleus
Q265: Which group 5A element is most metallic?<br>A)N<br>B)P<br>C)Sb<br>D)Bi
Q266: Sodium metal and water react to form
Q267: What is the charge on the Sc