Multiple Choice
Assume that the mixture of substances in drawing (1) undergoes a chemical reaction.Which of the drawings (2) -(4) represents a product mixture that is consistent with the law of mass conservation? 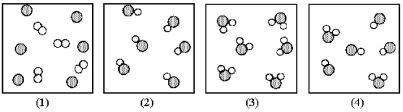
A) drawing (2)
B) drawing (3)
C) drawing (4)
Correct Answer:

Verified
Correct Answer:
Verified
Q2: How many electrons are in a neutral
Q3: The number of electrons in the ion
Q4: Which of the following elements is a
Q5: Elements in a periodic group have similar<br>A)chemical
Q6: What is the chemical formula for iron(II)phosphate?<br>A)Fe<sub>2</sub>P<br>B)Fe<sub>2</sub>PO<sub>4</sub><br>C)Fe<sub>3</sub>P<sub>4</sub><br>D)Fe<sub>3</sub>(PO<sub>4</sub>)<sub>2</sub>
Q8: Which of the compounds,Ca<sub> </sub>H<sub>2</sub>,H<sub>2</sub>O<sub> </sub>,C<sub> </sub>H<sub>4</sub>,XeF<sub>4</sub>
Q9: The symbol of the isotope having Z
Q10: Which subatomic particle has the smallest mass?<br>A)a
Q11: Mendeleev arranged the elements according to<br>A)atomic number
Q12: The solid compound,Na<sub>4</sub>SiO<sub>4</sub>,contains<br>A)Na<sup>+</sup>,Si<sup>4+</sup>,and O<sup>2-</sup> ions.<br>B)Na<sup>+</sup> ions and