Multiple Choice
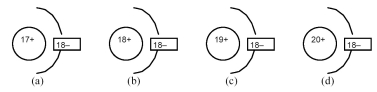
-Which of the above drawings represents a K+ ion?
A) drawing (a)
B) drawing (b)
C) drawing (c)
D) drawing (d)
Correct Answer:

Verified
Correct Answer:
Verified
Q239: The charge to mass ratio of an
Q240: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB4940/.jpg" alt=" -What is the
Q241: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB4940/.jpg" alt=" -What is the
Q242: The compound,Cu(I<sub> </sub>O<sub>3</sub> )<sub>2</sub>,is named<br>A)copper iodate(II).<br>B)copper(I)iodate.<br>C)copper(I)iodate(II).<br>D)copper(II)iodate.
Q243: What group of elements does the shaded
Q245: The number of electrons in the ion
Q246: Which horizontal row of the periodic table
Q247: What type of bonding is found in
Q248: Use the periodic table below to answer
Q249: Which of the following drawings represents a