Multiple Choice
. 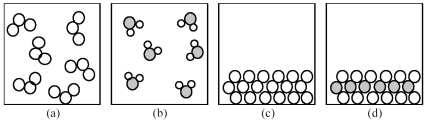
-If shaded and unshaded spheres represent atoms of different elements,which of the above drawings most likely represents an ionic compound at room temperature and a pressure of 1 atm?
A) drawing (a)
B) drawing (b)
C) drawing (c)
D) drawing (d)
Correct Answer:

Verified
Correct Answer:
Verified
Q118: A sample of pure calcium fluoride with
Q119: The formula of thallium(III)selenide contains _ thallium(III)and
Q120: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB4940/.jpg" alt=" -Which of the
Q121: Which of the compounds,Na<sub>3</sub>P,PH<sub>3</sub>,C<sub>2</sub>H<sub>6</sub>,IBr<sub>3</sub>,are ionic compounds?<br>A)only C<sub>2</sub>H<sub>6</sub><br>B)only
Q122: In the following drawings,shaded spheres represent cations
Q124: The number of neutrons in a neutral
Q125: The observation that 15.0 g of hydrogen
Q126: A sample of pure calcium fluoride with
Q127: Give the molecular formula corresponding to the
Q128: Rubidium belongs to the _ group of