Multiple Choice
The following diagram represents the reaction of A2 (unshaded spheres) with B (shaded spheres) .What is the balanced chemical equation for this reaction,and what is the limiting reactant? 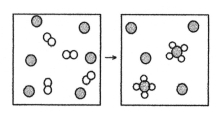
A) 2A2 + B → A4B;A2 is the limiting reactant.
B) 2A2 + B → A4B;B is the limiting reactant.
C) 4A2 + 6B → 2A4B;A2 is the limiting reactant.
D) 4A2 + 6B → 2A4B;B is the limiting reactant.
Correct Answer:

Verified
Correct Answer:
Verified
Q37: Consider two reactants,A and B.The molar mass
Q38: 1.00 mole of O<sub>2</sub> contains the same
Q39: Which one of the following is an
Q40: The following diagrams represent the reaction of
Q41: Dinitrogen monoxide gas decomposes to form nitrogen
Q43: What is the empirical formula of benzene,C<sub>6</sub>H<sub>6</sub>?
Q44: Which conducts electricity?<br>A)a large collection of iron
Q45: How many moles of H<sub>2</sub>O are needed
Q46: The balanced equation for the reaction of
Q47: In the reaction between glucose and oxygen,10.0