Multiple Choice
The following diagram represents the reaction of A2 (unshaded spheres) with B (shaded spheres) .How many moles of product can be produced from the reaction of 1.0 mol of A2 and 1.0 mol of B? 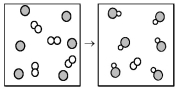
A) 0.5 mol of product
B) 1.0 mol of product
C) 3.0 mol of product
D) 6.0 mol of product
Correct Answer:

Verified
Correct Answer:
Verified
Q26: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB4940/.jpg" alt=" -2-Propanol has the
Q27: The balanced equation for the gaseous state
Q28: How many grams of the excess reagent
Q29: Each molecule of cortisone contains 21 atoms
Q30: A balanced equation has the same numbers
Q32: How many moles of BCl<sub>3</sub> are needed
Q33: What is the molar mass of iodine?<br>A)126.9
Q34: How many sulfate ions are there in
Q35: 1.00 mole of O<sub>2</sub> contains the same
Q36: How many sodium atoms are in 3.00