Multiple Choice
Assume that an aqueous solution of a cation,represented by shaded spheres,is allowed to mix with a solution of an anion,represented by unshaded spheres.Three possible outcomes are represented by boxes (a) -(c) . 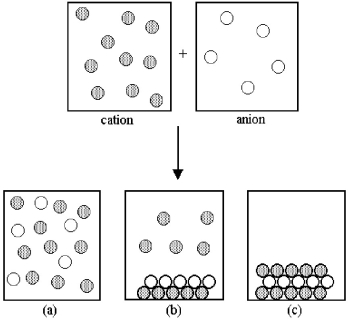
-Which outcome corresponds to the mixing of potassium and sulfide ions shown in the following equation? 2 K+(aq) + S2-(aq) → ?
A) box (a)
B) box (b)
C) box (c)
D) None of these
Correct Answer:

Verified
Correct Answer:
Verified
Q190: Balance the chemical equation given below,and determine
Q191: What is the oxidation number of the
Q192: When dissolved in water,NaOH behaves as<br>A)an acid
Q193: Which of the following compounds is an
Q194: Which of the compounds of H<sub> </sub>NO<sub>2,</sub>
Q196: Using the following sequence of reactions,determine the
Q197: Predict the products of a reaction between
Q198: What reagent could be used to separate
Q199: How many milliliters of 0.100 M FeCl<sub>3</sub>
Q200: How many grams of KNO<sub>3</sub> are needed