Multiple Choice
The following pictures represent aqueous solutions of three acids HA (A = X,Y,or Z) ,with water molecules omitted for clarity.Unshaded spheres represent hydrogen atoms or ions and gray spheres represent A atoms or ions.Which of the three is the strongest acid,and which is the weakest? 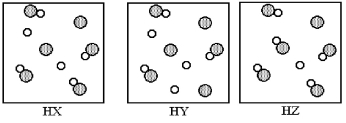
A) HX is the strongest acid and HY is the weakest acid.
B) HY is the strongest acid and HX is the weakest acid.
C) HY is the strongest acid and HZ is the weakest acid.
D) HZ is the strongest acid and HY is the weakest acid.
Correct Answer:

Verified
Correct Answer:
Verified
Q77: Write a balanced net ionic equation for
Q78: Assume that the conductivity of a solution
Q79: H<sub> </sub>Br<sub> </sub>,HCl<sub> </sub>,H<sub> </sub>ClO<sub>4</sub>,K<sub> </sub>Br<sub> </sub>,and
Q80: The mixing of which pair of reactants
Q81: What reagent could be used to separate
Q83: Using the following sequence of reactions,determine the
Q84: The substance is undergoing oxidation in the
Q85: What is the molarity of a solution
Q86: What is the concentration of an AlCl<sub>3</sub>
Q87: What is the molarity of chloride ions