Multiple Choice
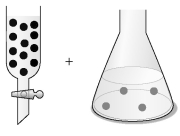
-The concentration of an aqueous solution of NaOCl can be determined by a redox titration with iodide ion in acidic solution: OCl- (aq) + 2 I- (aq) + 2 H⁺ (aq) → Cl⁻ (aq) + I2 (aq) + H2O (l)
Assume that the black spheres in the buret represent I⁻ ions,the gray spheres in the flask represent OCl⁻ ions,the concentration of the I⁻ ions in the buret is 0.120 M,and the volumes in the buret and the flask are identical.What is the concentration of the NaOCl in the flask,and what fraction of the I⁻ solution in the buret must be added to the flask to react with all the OCl⁻ ions?
A) 0.0400 M NaOCl;1/3 of the I⁻ must be added.
B) 0.0400 M NaOCl;2/3 of the I⁻ must be added.
C) 0.0600 M NaOCl;1/3 of the I⁻ must be added.
D) 0.0600 M NaOCl;2/3 of the I⁻ must be added.
Correct Answer:

Verified
Correct Answer:
Verified
Q39: The balanced net ionic equation for the
Q40: Write a balanced net ionic equation for
Q41: The chemical formula for iodous acid is<br>A)H<sub>
Q42: What is the concentration of NO<sub>3</sub><sup>-</sup> ions
Q43: Which outcome corresponds to the mixing of
Q45: Which outcome corresponds to the combination of
Q46: Which element will not react with liquid
Q47: The reaction Na<sub>3</sub>PO<sub>4</sub>(aq)+ 3 AgNO<sub>3</sub>(aq)→ Ag<sub>3</sub>PO<sub>4</sub>(s)+ 3
Q48: Disproportionation is a reaction in which a
Q49: How many grams of CaCl<sub>2</sub> are formed