Multiple Choice
Box (1) represents 1.0 mL of a solution of particles at a given concentration. 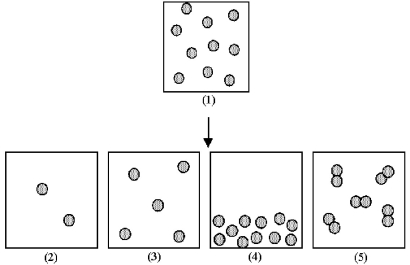
-Which of the boxes (2) -(5) represents 1.0 mL of the solution that results after (1) has been diluted by adding enough solvent to make 2.0 mL of solution?
A) box (2)
B) box (3)
C) box (4)
D) box (5)
Correct Answer:

Verified
Correct Answer:
Verified
Q105: What reagent could not be used to
Q138: The reaction Sr(NO<sub>3</sub>)<sub>2</sub>(aq)+ Cs<sub>2</sub>SO<sub>4</sub>(aq)→ SrSO<sub>4</sub>(s)+ 2 CsNO<sub>3</sub>(aq)is
Q139: What volume of a 0.540 M KOH
Q141: When 125 mL of 0.500 M AgNO<sub>3</sub>
Q142: If 100.mL of 0.200 M Na<sub>2</sub>SO<sub>4</sub> is
Q144: When 200.mL of 1.50 × 10<sup>-4</sup> M
Q145: A student prepared a stock solution by
Q146: Which of the following is not a
Q147: Predict the products of a reaction between
Q148: When 50.0 mL of a 1.00 M