Multiple Choice
Box (1) represents 1.0 mL of a solution of particles at a given concentration. 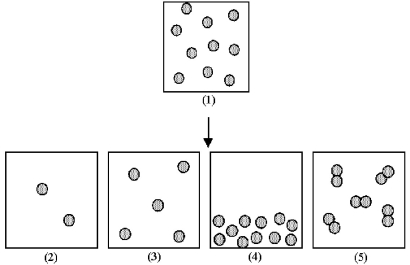
-Which of the boxes (2) -(5) represents 1.0 mL of the solution that results after (1) has been diluted by adding enough solvent to make 5.0 mL of solution?
A) box (2)
B) box (3)
C) box (4)
D) box (5)
Correct Answer:

Verified
Correct Answer:
Verified
Q67: How many milliliters of a 9.0 M
Q68: What is the concentration of FeCl<sub>3</sub> in
Q69: Based on the activity series,which metal dissolves
Q70: Using the following portion of the activity
Q71: What is the molar concentration of sulfate
Q73: How many milliliters of 0.260 M Na<sub>2</sub>S
Q74: Which pair of compounds is insoluble in
Q75: The reaction shown below is classified as
Q76: The number of moles of CaCl<sub>2</sub> in
Q77: Write a balanced net ionic equation for