Multiple Choice
Assume that the unshaded spheres in the buret represent H+ ions,the shaded spheres in the flask represent OH- ions,and you are carrying out a titration of the base with the acid. 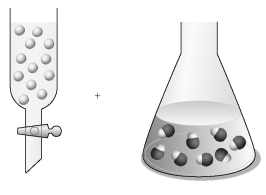
-If the volumes in the buret and the flask are identical and the concentration of the acid in the buret is 0.250 M,what is the concentration of the base in the flask?
A) 0.167 M
B) 0.250 M
C) 0.375 M
D) 0.667 M
Correct Answer:

Verified
Correct Answer:
Verified
Q201: What is the molar concentration of sodium
Q202: Assuming complete dissociation,the molar concentration of Br<sup>-</sup>
Q203: Using the following portion of the activity
Q204: The combustion reaction CH<sub>4</sub>(g)+ 2 O<sub>2</sub>(g)→ CO<sub>2</sub>(g)+
Q205: Which species functions as the oxidizing agent
Q207: Which statement about diluted solutions is false?
Q208: The hydrogen ion,H<sup>+</sup>,is also referred to as
Q209: The name of H<sub>2</sub>SO<sub>3</sub> is<br>A)sulfurous acid.<br>B)sulfuric acid.<br>C)hydrosulfuric
Q210: Write a balanced net ionic equation for
Q211: The acids HNO<sub>3</sub> and HNO<sub>2</sub> are named