Multiple Choice
What is the de Broglie wavelength of an electron (m = 9.11 × 10-31 kg) moving at a velocity of 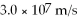 (10% of the speed of light) ?
(10% of the speed of light) ?
A) less than 3.9 × 10-12 m
B) 2.4 × 10-11 m
C) 3.3 × 10-8 m
D) greater than 1.1 × 10-4 m
Correct Answer:

Verified
Correct Answer:
Verified
Q120: The spheres below represent atoms of Sb,As,P,and
Q121: Which orbital-filling diagram represents the ground state
Q122: The spheres below represent atoms of Li,Be,B,and
Q123: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB4940/.jpg" alt=" -Atoms of which
Q124: According to the Balmer-Rydberg equation,which transition results
Q126: What are the possible values of l
Q127: What is the deBroglie wavelength in meters
Q128: Molecular vibrational energy transitions are observed in
Q129: What is the ground-state valence-shell electron configuration
Q130: Light behaves as if it were a