Multiple Choice
For the fourth-shell orbital shown below,what are the principal quantum number,n,and the angular momentum quantum number,l? 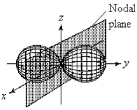
A) n = 4 and l = 0
B) n = 4 and l = 1
C) n = 4 and l = 2
D) n = 4 and l = 3
Correct Answer:

Verified
Correct Answer:
Verified
Q82: An electron in a 4p orbital can
Q83: Two electromagnetic waves are represented below. <img
Q84: The element Ga has how many valence
Q85: Compared to Si ,Cl has a _
Q86: For the fourth-shell orbital shown below,what are
Q88: The symbol [Kr] represents<br>A)4s<sup>2</sup>4p<sup>6</sup>.<br>B)1s<sup>2</sup>2s<sup>2</sup>2p<sup>6</sup>3s<sup>2</sup>3p<sup>6</sup>4s<sup>2</sup>4p<sup>6</sup>.<br>C)1s<sup>2</sup>2s<sup>2</sup>2p<sup>6</sup>3s<sup>2</sup>3p<sup>6</sup>3d<sup>10</sup>4s<sup>2</sup>4p<sup>6</sup>.<br>D)1s<sup>2</sup>2s<sup>2</sup>2p<sup>6</sup>3s<sup>2</sup>3p<sup>6</sup>3d<sup>10</sup>4s<sup>2</sup>4p<sup>6</sup>4d<sup>10</sup>.
Q89: According to the Balmer-Rydberg equation,electromagnetic radiation with
Q90: The absorption of a photon of wavelength
Q91: For hydrogen,what is the wavelength of the
Q92: A baseball with a mass of 150