Multiple Choice
For the fourth-shell orbital shown below,what are the principal quantum number,n,and the angular momentum quantum number,l? 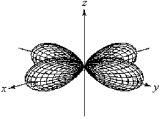
A) n = 4 and l = 0
B) n = 4 and l = 1
C) n = 4 and l = 2
D) n = 4 and l = 3
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q50: According to the Heisenberg uncertainty principle,<br>A)the position
Q51: The element Bh has how many valence
Q52: Which orbital-filling diagram represents the anomalous ground
Q53: The amount of data that can be
Q54: A neutral N atom has how many
Q56: For the fourth-shell orbital shown below,what are
Q57: The ground-state electron configuration of the C<sup>4-</sup>,is
Q58: The laser used to read Blu-Ray discs
Q59: Which element has the ground-state electron configuration
Q60: What are the possible values of l